PRECLINICAL ASSAYS
Identifying potential toxicity at early research stages allows to optimize new compounds development
REQUEST MORE INFO
Cell-based assays with high flexibility and a ready-to-use system.
Full cell functionality after transportation.
Validation data available and high quality-control standards.
Caco-2 cell line
Drug-induced toxicity, especially in the liver, kidneys and gastrointestinal tract, poses a significant risk that can lead to cellular damage and organ failure.
While preclinical studies mostly rely on animal experimentation as the standard for drug safety evaluation, in vitro cytotoxicity assays provide a reliable alternative for early toxicity screening. These assays offer key advantages, including ethical compliance, cost-effectiveness and enhanced efficiency in detecting potential toxic effects during the drug development process:
CELL TOXICITY ASSAY
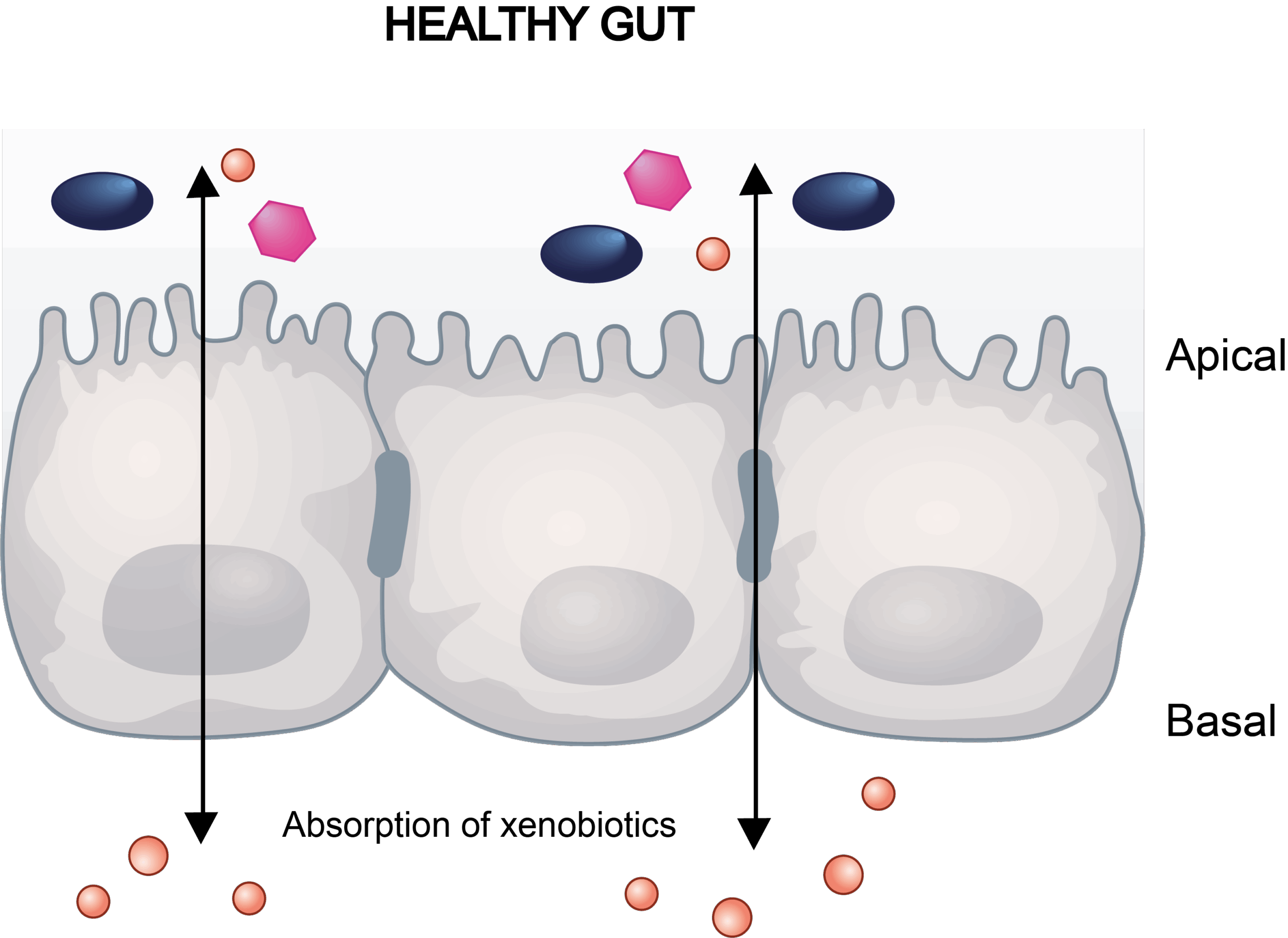
Drug-induced gastrointestinal (GI) toxicity is one of the most commonly observed clinical adverse events that can manifest with varying degrees of severity, from mild discomfort such as nausea and constipation to more serious conditions like diarrhea and abdominal pain.
In severe cases, these toxic effects can lead to drug withdrawals from the market, underscoring the need for reliable early-stage screening methods.
Conventional 2D monocultures of intestinal cell lines, such as Caco-2 cells, serve as well-established in vitro models for evaluating drug-induced GI toxicity. These models enable researchers to evaluate the cytotoxic effects of compounds and their impact on intestinal barrier integrity.
Upon differentiation, Caco-2 cells form a monolayer that mimics key properties of the intestinal epithelium, providing a reliable platform to study compound-induced cytotoxicity, by measuring cell viability or proliferation, morphological evaluation and cell function assessment.
OUR PRODUCTS
The CacoReady Multiwell plate enables high-throughput cytotoxicity screening using standard protocols or commercially available methodologies, including the evaluation of cellular damage through morphological changes, assessment of cell viability, measuring cell growth and metabolic activity.
In these assays, Caco-2 cells are exposed to test compounds, followed by an incubation period, after which a specific marker is measured to determine the number of viable cells relative to positive controls (including cytotoxic effects) and negative controls (vehicle).
Commonly cytotoxicity evaluation methods include:
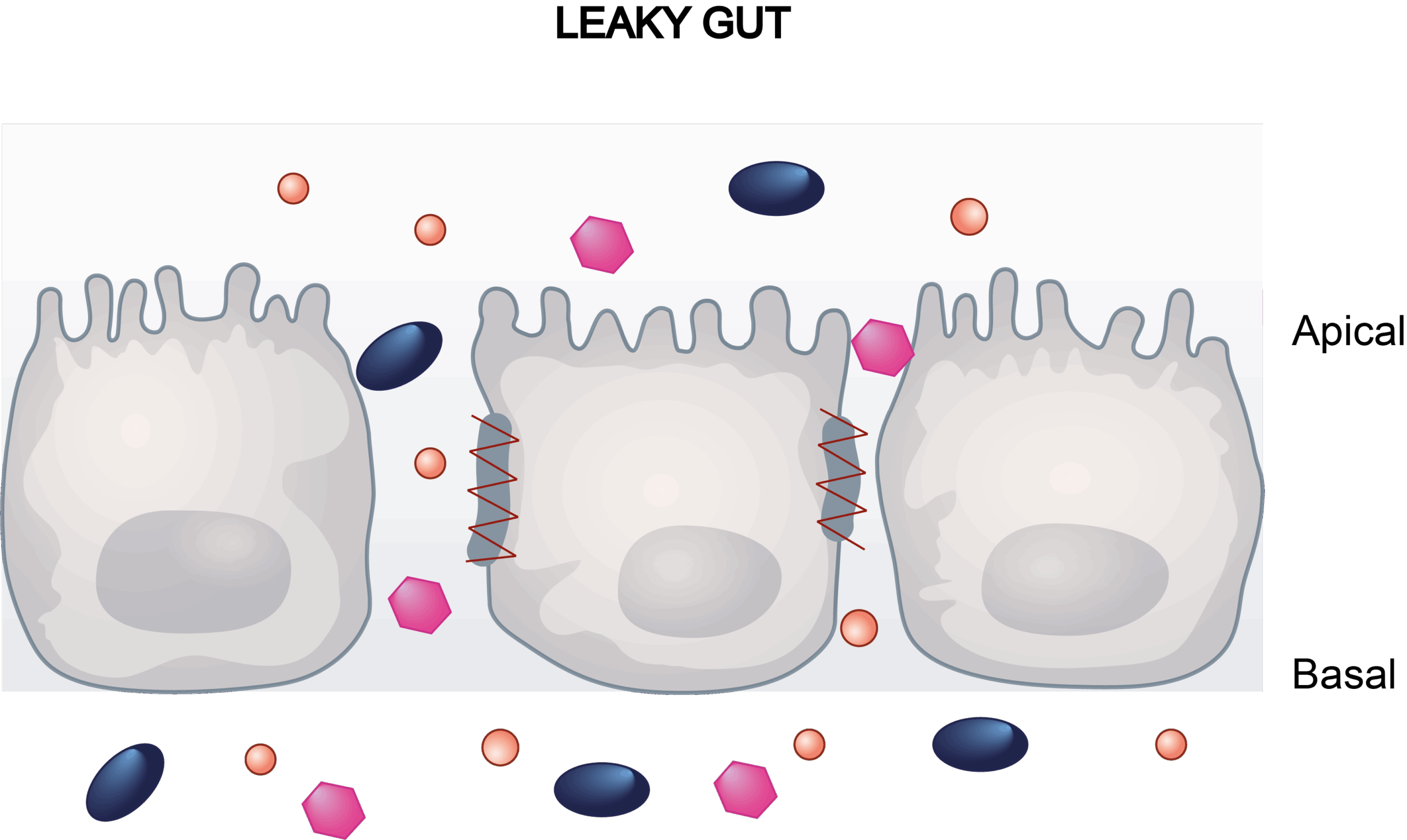
Gastrointestinal toxicity is closely linked to the disruption of tight junction proteins, which regulate intestinal permeability and maintain the epithelial barrier. Damage to these proteins, often caused by drugs, toxins, or disease conditions leads to increased permeability and compromised barrier function. Adverse outcomes such as drug-induced diarrhea often correlate with this barrier dysfunction, underscoring the importance of in vitro assays for early toxicity screening.
Our Caco-2 transwell model addresses this need by integrating trans-epithelial electrical resistance (TEER) and lucifer yellow (LY) paracellular flux assays to quantify barrier dynamics.
Read the assay
Download the application notes
Caco-2 cell line
Beyond gastrointestinal toxicity, we are advancing our research into novel in vitro cytotoxicity models to enhance drug safety assessment.
Our key areas of interest focus on human-relevant toxicity insights across critical organ systems:
Our ongoing research efforts aim to expand our portfolio with ready-to-use in vitro toxicity cell-based plates, supporting early-stage drug screening. We are also exploring collaborative opportunities to advance the creation of specialized models.
Explore our latest advancements in hepatotoxicity, cardiotoxicity, and other emerging models in our Research Areas.
The integrated Transwell format allows apical-basal polarization, making it ideal for toxicity tests by studying membrane resistance, allowing a stratification of compound toxicity. Non-Transwell plates (CacoReady multiwell) are used for traditional toxicity tests, such as colorimetric tests, which did not require polarized cells. Individual Transwells are suitable for assays requiring flexibility in handling each insert independently for varied time points or measurements.
According to the European and the American Regulatory Agencies (EMA and FDA), acute toxicity studies are recommended for pharmaceuticals intended for human use. These guidelines emphasize the importance of in vitro cytotoxicity assays in early-stage drug development.
As a supplier, we primarily provide assay plates but do not offer testing services directly. However, we can connect you with trusted partners who specialize in conducting external assays using our products. In specific cases, we are open to collaborations to explore new applications of mutual interest.
For non-Transwell plates, you can perfom the assay as needed using standard available methods for toxicity testing. In particular, AlamarBlue, MTT, and WST colorimetric reagents are validated for compatibility with CacoReady plates.
To ensure assay robustness and reproducibility, it is recommended to validate the selected method for each specific cell type using appropriate positive controls. Additionally, potential reagent-specific cytotoxic effects should be carefully evaluated to maintain assay reliability and accuracy.
You can find more information regarding cytotoxicity analytical methods here.
This assay utilizes Transepithelial Electrical Resistance (TEER) and Lucifer Yellow (LY) flux to assess monolayer integrity. TEER provides real-time monitoring of monolayer integrity, while LY flux directly measures paracellular permeability, enabling dual validation of tight junction functionality. This approach outperforms traditional methods by capturing both structural and functional changes in the epithelial barrier, offering a predictive tool for stratifying diarrhea risk associated with therapeutics.
You can learn more from our cytotoxicity assay protocol.
The reference compounds used in these assays are Nadolol, Quinidine, Verapamil, and Gemfibrozil. In the transwell system, Gemfibrozil is stratified as cytotoxic, Verapamil and Quinidine are described as moderate toxicants, and Nadolol is non-toxic.
Yes, quality control is batch-specific and it is included with every kit to provide customers with a reference before and after shipment.
No, the Shipping Medium consists of a semi-solid culture system specifically designed to preserve cells at room temperature (15-25ºC). This medium maintains a suitable physicochemical environment, keeping adequate moisture conditions for cellular homeostasis and forming a protective cushion that protects cell integrity and functionality during long-distance shipments and up to seven days.
Biotechnology company specialized in the development and commercialization of innovative solutions for the pharmaceutical industry and biomedical research.
