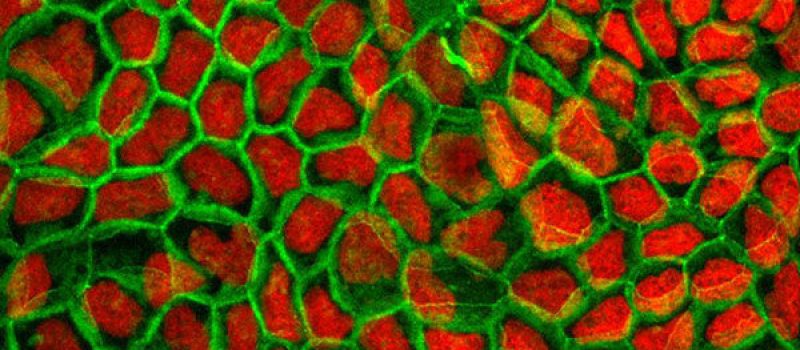
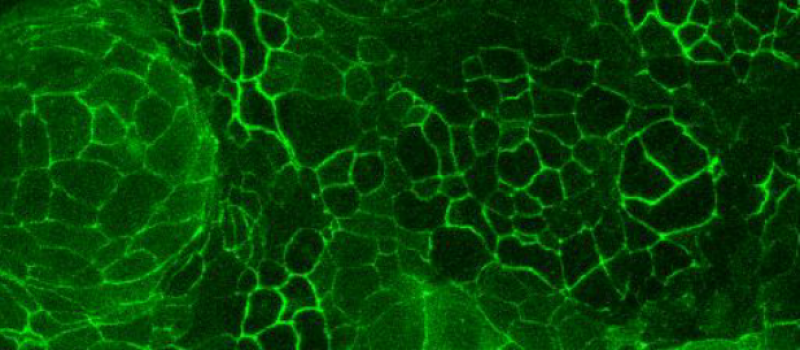
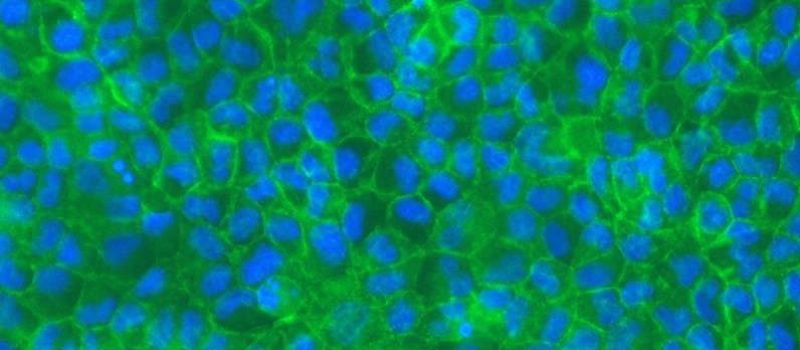
PRECLINICAL ASSAYS
In Vitro permeability testing for drug development
The use of our products manufactured with Caco-2, HT-29 and MDCKII cells for permeability assays will help you to investigate your compound absorption across a relevant barrier.
Request more information
Cell-based assays with high flexibility and a ready-to-use system.
Highly predictive and useful in the in vitro to in vivo progression.
Worldwide shipments at room temperature thanks to our patented technology.
CELL PERMEABILITY ASSAY MODELS
Ready-to-use cells for preclinical stages
Caco-2 Permeability Assay
The CacoReady plate contains Caco-2 cells, a human colorectal cancer-derived cell line that has proven to successfully mimic the intestinal epithelial barrier.
READ MORE
For over thirty years, Caco-2 cells have been the gold standard for evaluating oral drug absorption. In this in vitro model, Caco-2 cells are seeded and differentiated over 21 days on transwell plates with permeable membrane supports, forming apical and basal compartments that mimic the intestinal lumen and bloodstream.
Initially used to assess the permeability of passively transported molecules, Caco-2 cells also express key efflux transporters like Multidrug Resistance Protein 1 (MDR1) and Breast Cancer Resistance Protein (BCRP), making them a valuable tool for studying active drug transport.
Widely applied in early drug development, this model helps predict human intestinal absorption. Regulatory guidelines also recommend its use to classify drugs according to the Biopharmaceutical Classification System (BCS) and for conducting transporter-mediated drug-drug interaction (DDI) studies.
CacoReady is available in 24-well and 96-well formats, offering a ready-to-use solution for high-throughput drug permeability assays.
Caco-2 and HT-29 Permeability Assay
The CacoGoblet model results from coculturing Caco-2 and HT29-MTX cells, two human colon cancer-derived cell lines, that together form a physiologically relevant intestinal epithelial barrier.
READ MORE
CacoGoblet combines absorptive (Caco-2) and mucus-secreting (HT29-MTX) cells, creating a model with physiological intestinal properties that differ from standard Caco-2 cultures. This co culture develops a protective mucus layer lining on top of the cells, forms a barrier that is less tight than pure Caco-2 cultures, and exhibits electrophysiological properties closer to those of the human small intestine.
This product aligns with the evolving landscape of in vitro models, complementing regulatory-standard Caco-2 assays for drug absorption studies. Recognized for its physiological relevance, it provides valuable insights into passive diffusion and active transport, often revealing higher permeability, particularly for low-permeability compounds.
In addition to drug permeability testing, CacoGoblet responds well to inflammatory mediators, providing valuable information on the efficacy of anti-inflammatory drugs.
CacoGoblet is available in a 24-well plate format, offering a ready-to-use solution for high-throughput screening.
MDCKII Permeabilty Assay
Madin-Darby Canine Kidney Type II (MDCKII) cells, included in our PreadyPort plates, provide a relevant in-vitro model to assess drug permeability by passive diffusion across a relevant barrier.
READ MORE
MDCKII cells originate from normal kidney tissue of an adult dog. When seeded and differentiated for 11 days on transwell plates with permeable membrane supports, these cells develop a polarized morphology with distinct brush-border structures, apical cell-cell junctions, and lateral spaces. They also establish a paracellular permeation route controlled by tight junctions. MDCKII monolayers are classified as “leaky epithelia” due to their low transepithelial electrical resistance (TEER).
MDCKII support both transient and stable gene transfection, making them especially relevant for predicting in vivo drug brain penetration and transported-mediated drug-drug interactions. Their versatility makes them widely used in permeability research.
In addition, this model has been used to characterize paracellular transport, including tight junction proteins (e.g., claudin, occludin), the effects of osmotic changes, ion and size selectivity, barrier tightness, and morphological and physiological changes under different environmental conditions, or to elucidate paracellular transport mechanisms.
PreadyPort WT is available in 24-well and 96-well plate formats, offering a flexible, ready-to-use solution for high-throughput screening.
Compound's suitability for oral dosing
Explore ReadyCell Kits
Cell models that replicate the human intestinal barrier are a well-established in vitro technique for screening oral absorption during drug discovery and development.
Our range of pre-plated transwell products allows the rapid and accurate determination of drug transport across Caco-2, HT-29, and MDCKII cell monolayers, enabling you to evaluate drug transporters efficiently.
Permeability Assay Protocol
Transporter-mediated drug interactions protein is assessed by conducting uni- and bidirectional assays across the cell monolayers grown in the Transwell diffusion cell system.
Explore the detailed guide for conducting the Caco-2 permeability test procedure, including reference values and key insights, with the option to download the application notes.
FAQs: Efflux transporters and drug-interaction studies
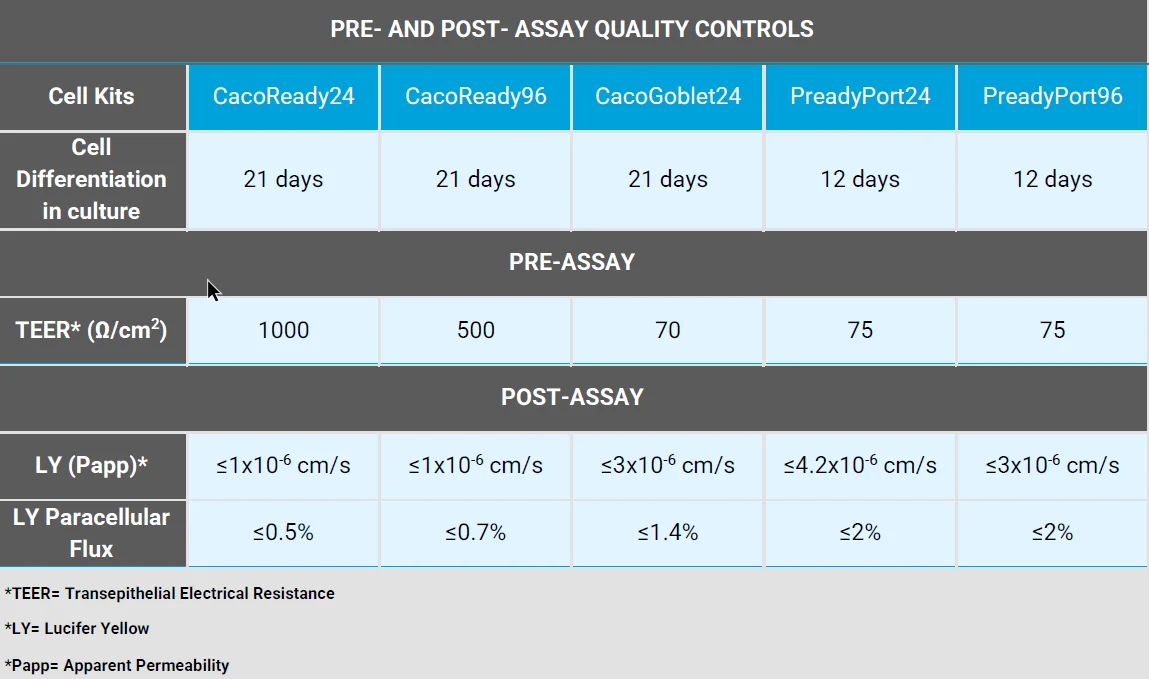
What are the quality controls for our transwell cell systems?
Is the quality control data specific for each kit?
What is the difference between CacoReady and CacoGoblet?
CacoReady and CacoGoblet are two cell-based kits for in vitro assaying drug intestinal permeability. CacoReady contains absorptive epithelial cells (Caco-2 cells), while CacoGoblet is the result of coculturing Caco-2 cells and mucus-secreting cells (HT29-MTX cells), bringing it closer to the most physiological conditions.
The barrier properties also differ because they include diverse cell phenotypes: very tight for CacoReady with TEER values ≥ 1000 ohms x cm2 and looser for CacoGoblet (≥ 70 ohms x cm2). Drug permeability assays for both models are very consistent.
What do the current regulations establish on the basis of permeability tests?
Does ReadyCell shipping medium affect cell lines
No, the Shipping Medium consists of a semi-solid culture system specifically designed to preserve cells at room temperature (15-25ºC). This medium maintains a suitable physicochemical environment, keeping adequate moisture conditions for cellular homeostasis and forming a protective cushion that protects cell integrity and functionality during long-distance shipments and up to seven days.
Is it possible to add a testing service to the order?
As a general rule, we act as a supplier and do not provide testing services. Nonetheless, feel free to contact us if you wish to test our plates externally. We can direct you to our partners who can assist you in conducting the assay effectively. Additionally, in certain situations, we are open to collaborating to try out new applications of interest to both of us.
What are the differences between transwell, no-transwell and individual transwell?
Transwell cell culture inserts are recommended by reference to perform permeability studies since they enable apical-basal polarization. Cell models are offered on 24- and 96-inserts connected by a rigid plate that allows being handled as a single unit or in individual transwell inserts. The former is compatible with cell incubation mechanization, while the rear format requires moving each well separately. Individual transwells are useful for assaying drug permeation kinetics and different drug exposure times. The non-transwell format is used for tests other than permeability studies, such as toxicity, DNA, RNA, protein isolation, and immunodetection.
For an effective permeability assay protocol, as well as a cytotoxicity assay protocol, it’s crucial to consider the choice of well system carefully.
Which are the main reference compounds for CacoReady and what are the differences between them?
The reference molecules used in the absorption studies are categorized as high, moderate and low-permeable compounds. Thus, compounds such as metoprolol and propranolol with apparent permeability (Papp) values >10 x 10-6 cm/s are highly absorbed. In contrast, metformin and amiloride are classified as moderately permeable, with Papp values ranging from 1 to 10 x 10-6 cm/s. Atenolol and inulin are low-permeable compounds, as their Papp values are less than 1 x 10-6 cm/s.
Additionally, some drugs may be potential substrates and inhibitors for efflux transporters that actively pump them out of cells. For instance, digoxin and quinidine serve as reference substrates for the multidrug resistance protein 1 (MDR1), while prazosin and dantrolene are substrates of the breast cancer resistance protein (BCRP). Drugs that are substrates for these efflux transporters typically exhibit efflux ratios greater than 2 when assessed using bidirectional transport assays across cellular monolayers.
For a more detailed explanation of these reference compounds and their differences, check out our in-depth post on reference compounds.