Understanding Food-Drug Interactions
Food intake significantly alters the conditions within the gastrointestinal tract, influencing drug absorption and bioavailability. Factors such as pH, motility, and microbiota composition can modify how drugs interact with the body, making food-drug interactions a critical concern in pharmacokinetics.
In addition to these changes, certain food components interact directly with drug metabolism and transport mechanisms, particularly through the CYP3A4 enzyme and various membrane transporters. These interactions can enhance or reduce drug efficacy, leading to potential therapeutic failures or adverse effects.
Key Mechanisms of Food-Drug Interactions
Food-drug interactions can occur through multiple pathways, including:
- Alteration of gastric and intestinal conditions – pH, motility, and enzymatic activity influence drug solubility and absorption.
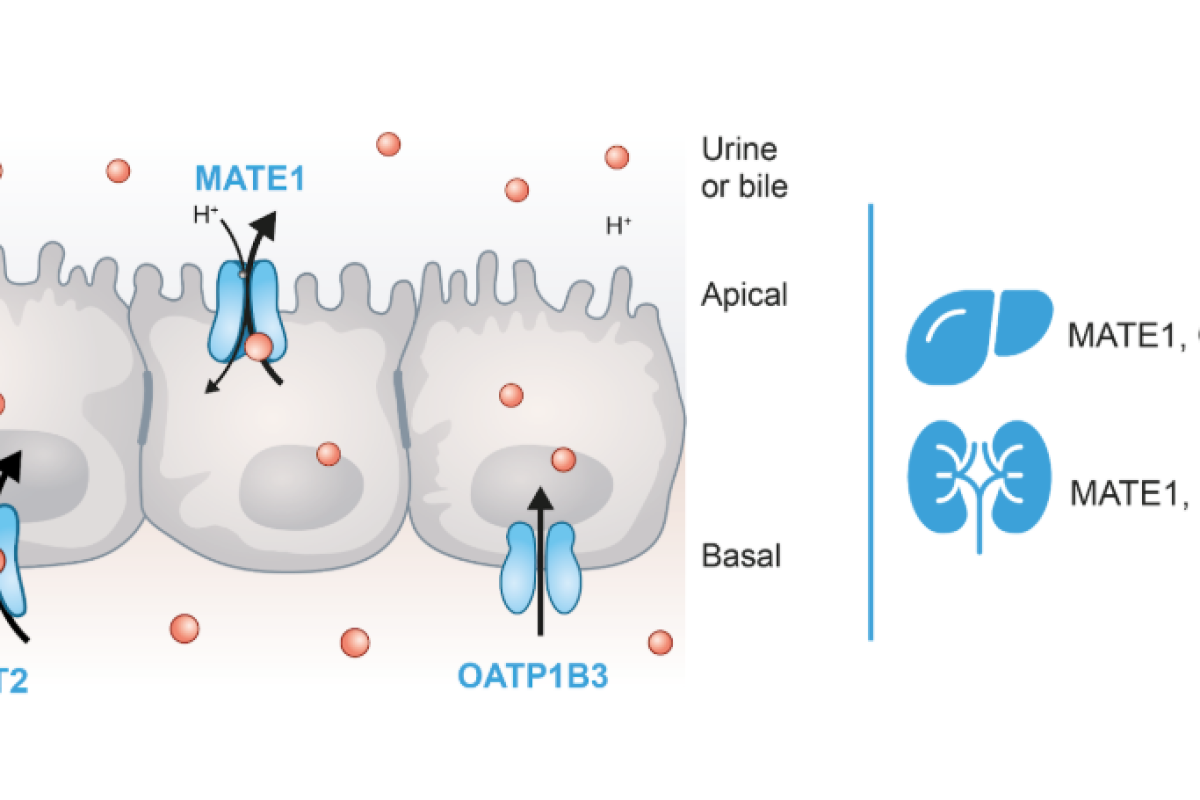
- Competition for membrane transporters – Nutrients and drugs may compete for uptake and efflux transporters such as:
- P-glycoprotein (Pgp)
- Breast cancer resistance protein (BCRP)
- Multidrug resistance-associated protein 2 (MRP2)
- Organic anion transporting polypeptide 2B1 (OATP2B1)
- Enzyme modulation – Food components can inhibit or induce metabolic enzymes like CYP3A4, affecting drug metabolism.
Notable Food-Drug Interactions
Research has documented various food components that affect drug transport and metabolism:
- Curcumin (food coloring agent) – Inhibits Pgp, affecting drug efflux.
- Citrus auraptene – Induces Pgp, potentially modifying drug bioavailability.
- Azo dyes (Allura Red AC, Brilliant Black BN) – Block BCRP and MRP2, influencing drug clearance.
- Neohesperidin dihydrochalcone (sweetener) – Inhibits BCRP, altering drug absorption.
- Flavonoids (Naringenin, Quercetin) – Reduce absorption of fexofenadine and celiprolol (OATP2B1 substrates).
Based on the adverse effects that can be derived from potential food-drug interactions, the FDA and EMA have published several guidances encouraging the pharmaceutical industry to research interactions during drug development. A deeper understanding of the modulation of efflux and uptake transporters by food intake will help us to better understand the inter- and intra-variability in the pharmacokinetics of drugs.
To this end, MedTech Barcelona has available several ready-to-use cell-based assays mimicking the intestinal barrier (Caco-2 cells) and cells of epithelial origin (MDCKII cells) individually overexpressing efflux transporters (e.g., Pgp (MDR1), BCRP). Cells are preplated and differentiated on transwell insert plates and delivered worldwide by means of our patented gel-like Shipping Medium.
For more information, contact our expert team at reagents@medtechbcn.com
Additional Readings
- Chabane MN et al. J Pharm Pharmacol, 2009, 61:1473-83 (https://doi.org/10.1211/jpp.61.11.0006)
- European Medicines Agency (EMA), 2012. Guideline on the Investigation of Drug Interactions. (Guideline PDF)
- Food and Drug Administration (FDA). Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies (FDA Guidance PDF)
- Koziolek M et al. European Journal of Pharmaceutical Sciences, 2019, 134:31-59 (https://doi.org/10.1016/j.ejps.2019.04.003)
- Nabekura T et al. Food Funct 2020, 11:5017-5023 (DOI: 10.1039/d0fo00315h)
- Sjöstedt N et al. Mol Pharm. 2017, 14:3824-3833 (DOI: 10.1021/acs.molpharmaceut.7b00563)