Ensuring the safety of new drugs is a crucial step in drug development to prevent the withdrawal of drugs from the market due to adverse side effects and toxicity. Traditional in vitro colorimetric methods—such as MTT, WST-1, or Alamar Blue—are widely used to assess cytotoxicity. However, these methods often detect toxicity too late, after significant cell damage has already occurred.
Are colorimetric in vitro assays sensitive enough for evaluating drug toxicity?
At MedTech Barcelona, we have explored a more sensitive and predictive approach using our CacoReady plates, which evaluate early indicators of cell damage in the intestinal barrier. By measuring transepithelial electrical resistance (TEER) and Lucifer Yellow (LY) paracellular flux, we can detect drug-induced effects before cell death occurs, helping to predict potential adverse reactions more effectively and even stratify intestinal drug toxicity (non-toxic, moderately toxic and very toxic).
How can early detection of drug toxicity improve drug safety?
Many drugs cause gastrointestinal side effects, but traditional colorimetric tests may not detect them accurately. Our study compared these conventional methods with CacoReady using reference compounds described as causing diarrhea with a known incidence of toxicity:
Comparison of colorimetric assays and early indicators of TEER and LY for cytotoxicity evaluation
- Colorimetric cell viability assay by Alamar Blue, for detecting metabolically active cells.
- Barrier Integrity Assay (TEER & LY Flux), conducted in CacoReady® plates following the procedure described in the permeability assay protocol.
| Acceptance criteria | CacoReady® 96w |
| TEER | > 500 Ω x cm2 |
| LY Paracellular Flux | ≤ 0.7% |
Results
- Gemfibrozil – detected as toxic in both models, but at a lower concentration when the cell barrier integrity is evaluated by TEER and LY flux as the endpoint.
- Quinidine and Verapamil (figure 2) – classified as moderately toxic but only detected as toxic with CacoReady® model (TEER and LY flux measurements).
- Nadolol – used as a control, confirmed as non-toxic in all tests.
These results are part of a study conducted by MedTech Barcelona R&D department and published in an application note.
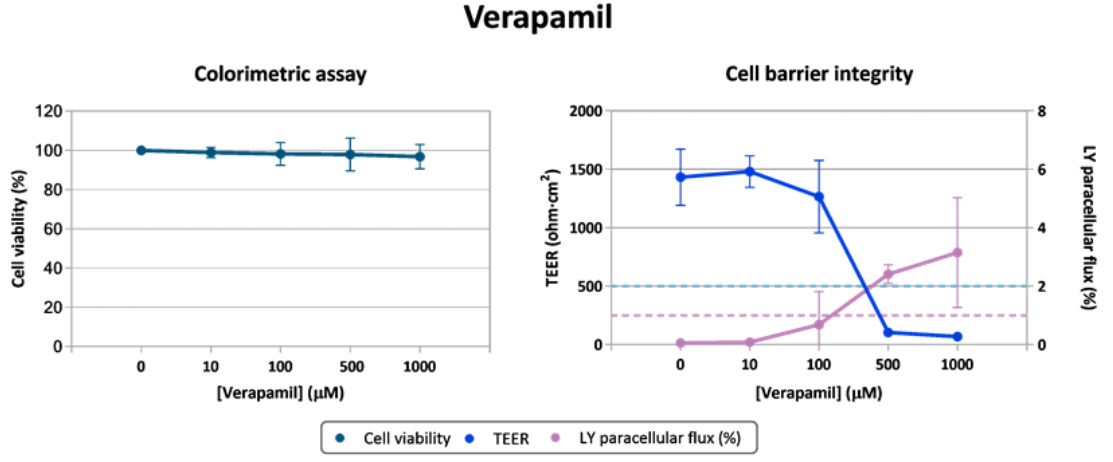
Figure 2. Evaluation of verapamil cytotoxicity in Caco-2 cell line.
Caco-2 cells seeded in a 96-well plate and in 96-well transwell inserts (CacoReady) were exposed to increasing concentrations of verapamil for a 24-h period. Alamar Blue was the colorimetric assay used to quantify metabolically active (living) cells (left panel) and TEER values and LY paracellular flux were the parameters used as indicators of cell barrier integrity (right panel).
These findings demonstrate that assessing cell barrier integrity, an early indicator of intestinal cytotoxicity, provides a more accurate prediction of drug-induced toxicity than traditional viability assays and enable the drug stratification.
Learn more
To learn more about CacoReady products used in cytotoxicity testing, please visit our web page.
If you need more detailed information about Caco-2 cytotoxicity studies, you can download the application notes on the MedTech Barcelona cytotoxicity study here. Alternatively, feel free to contact us through our online form or by emailing reagents@medtechbcn.com.