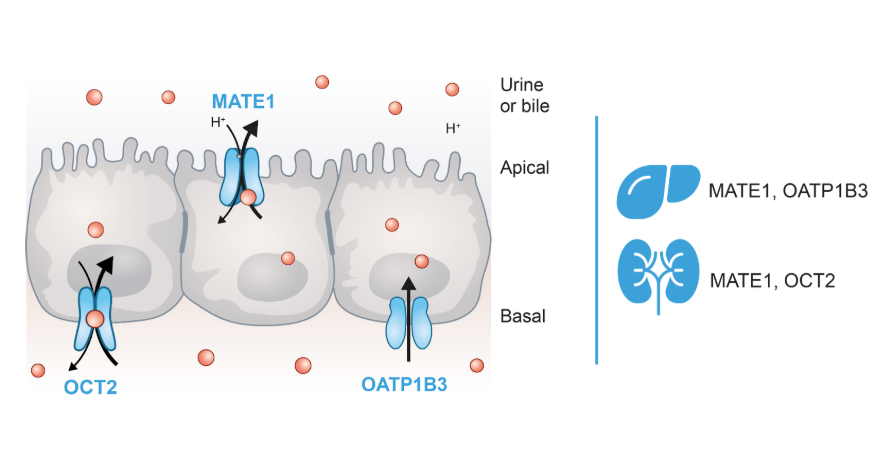
The liver and kidneys have clearly defined physiological functions in the body. They act as excretory organs for drugs, potentially toxic xenobiotics, and metabolic waste products such as polar metabolites.
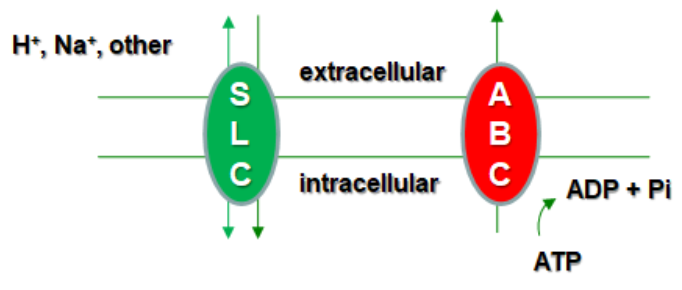
Therefore, two main pathways of drug elimination have been described: hepatic clearance, which includes both metabolic transformation and biliary excretion, and renal secretion. These processes, both hepatic and renal, are driven by the so-called membrane transporters of the solute transporter superfamily (SLC) and ATP-binding cassette (ABC) superfamily, strategically located in the proximal tubules of the kidneys and in the hepatocytes and bile canaliculi of the liver.
The main renal and hepatic transporters in humans
In total, more than 400 transporter genes have been identified within the SLC and ABC superfamilies, playing crucial roles in the movement of endogenous and exogenous compounds across biological membranes. However, only a subset of these transporters is considered key for hepatic and renal drug elimination, and regulatory agencies specifically recommend certain ones for in vitro studies to assess drug interactions and clearance mechanisms. The main transporters involved include the following:
- Multidrug and toxin excretion 1 (MATE1): MATE1 is highly expressed in the liver and the kidney located on the canalicular membrane (apical, bile side) of hepatocytes, and the brush-border membrane (apical, urine side) of proximal tubule cells. Under physiological conditions, it functions as an efflux transporter, mediating the elimination of diverse substrates, primarily organic cations, in the kidney and liver. However, in in vitro studies, it is commonly assessed as an uptake transporter due to the experimental conditions used to evaluate its activity.
- Organic cation transporters (OCTs): These uptake transporters include three different isoforms, with the OCT2 isoform mainly expressed in the kidney, on the basolateral membrane of the renal proximal tubule cells. It plays a key role in the disposition and renal clearance of mostly cationic drugs and endogenous compounds.
- Organic anion transporting polypeptide 1B3 (OATP1B3): OATP1B3 is an uptake transporter exclusively expressed in the liver on the sinusoidal (basolateral) side of centrilobular hepatocytes. It is responsible for the hepatic uptake of some important drug classes, notably statins, for the uptake of bile acids and bilirubin, as well as some other endogenous molecules.
- P-glycoprotein (P-gp or MDR1): Pgp is one of the most characterized efflux pumps. It is broadly expressed in secretory organs and tissue barriers, but also in normal tissues. In the kidney it is localized in the luminal or apical site of the proximal tubule epithelium and in the liver on the bile canalicular surface.
- Breast cancer resistance protein (BCRP): BCRP is highly expressed in barrier tissues such as the colon, small intestine, blood-brain barrier (BBB), placenta, and liver canalicular membrane. It is an efflux transporter that serves two major drug transport functions. Firstly, it restricts the distribution of its substrates into organs such as the brain, testes, placenta, and across the gastrointestinal tract (GIT). Secondly, it eliminates its substrates from excretory organs, mediating both biliary and renal excretion, and occasionally direct gut secretion.
The value of transporter expertise in DDI clinical trials
The industry is now becoming increasingly aware of the role of these transporters in clinically relevant drug-drug interactions (DDIs). Understanding the role of these transporters in the clearance of new molecular entities (NME) is normally performed in-vitro at the early stages of drug development. The reason lies in the possible alteration of the pharmacokinetics/pharmacodynamics (PK/PD) of these new drugs as well as those simultaneously administered.
Here are some examples of drug-drug interactions, which include a rough overview of substrates and inhibitors of major human renal and hepatic transporters.
| Transporter | Substrates | Inhibitors | Reference |
|---|---|---|---|
| P-gp | Digoxin | Ritonavir | Ding R et al., (2004) |
| BCRP | Topotecan | Elacridar | Kruijtzer CM et al., (2002) |
| Rosuvastatin | Cyclosporine | Simonson SG et al., (2004) | |
| OATP1B3 | Rosuvastatin | Cyclosporine | Simonson SG et al., (2004) |
| OCT2 | Metformin | Cimetidine | Somogyi A and Muirhead M (1987) |
| MATE1 | Metformin | Cimetidine | Somogyi A and Muirhead M (1987) |
| Procainamide | Cimetidine | Somogyi A et al., (1983) |
Figure 2: Examples of clinically relevant Drug-Drug Interactions
In order to ascertain in vitro whether NME are substrates or/and inhibitors of renal and hepatic drug transporters, MedTech Barcelona, through its main product line ReadyCell, offers several ready-to-use cell-based assays individually overexpressing efflux pumps and uptake transporters (e.g., MATE1, OCT2, OATP1B3, Pgp, or BCRP). Cells are preplated and delivered worldwide at room temperature using our patented gel-like Shipping Medium, maintaining cells in optimal conditions.
For more information, contact our expert team through our form or by sending an email to reagents@medtechbcn.com.
Additional readings
- U.S. Food and Drug Administration (FDA). Guidance for Industry. January 2020. FDA.gov
- Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;
53:503-529. doi:10.1146/annurev-pharmtox-011112-140317 - Lin, L., Yee, S., Kim, R. et al. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov 14, 543–560 (2015).
https://doi.org/10.1038/nrd4626 - Yin J, Wang J. Renal drug transporters and their significance in drug-drug interactions. Acta Pharm Sin B. 2016;6(5):363-373.
doi:10.1016/j.apsb.2016.07.013 - Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal Drug Transporters and Drug Interactions. Clin Pharmacokinet. 2017;56(8):825-892.
doi:10.1007/s40262-017-0506-8