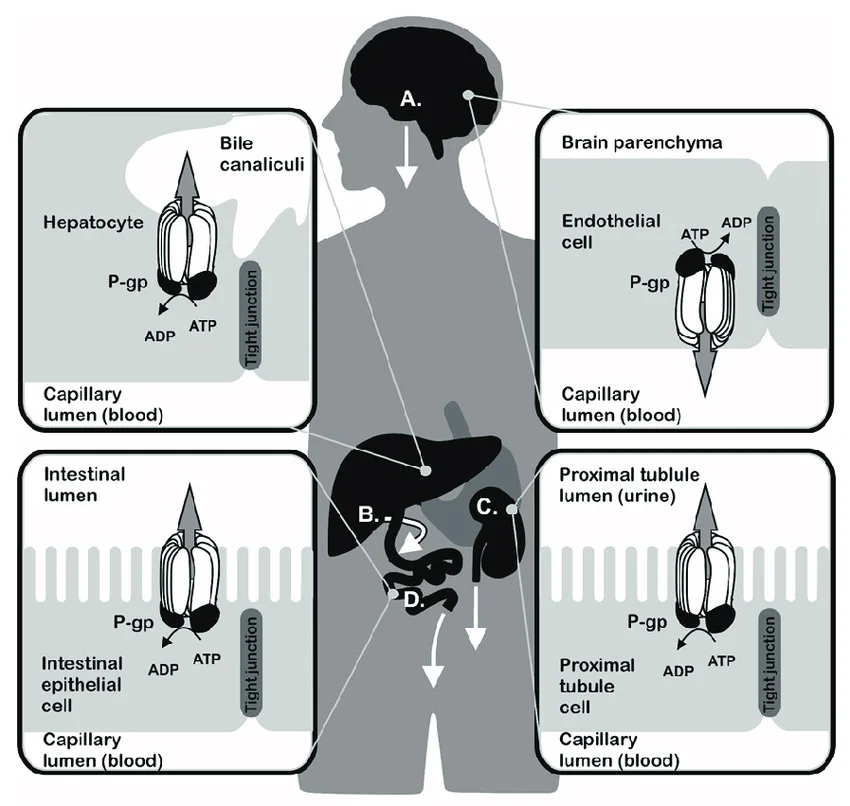
When it comes to preclinical drug development, understanding how a new compound interacts with key transporters is essential. The multidrug resistance protein 1, MDR1 (P-glycoprotein or P-gp), and the Breast Cancer Resistance Protein, BCRP, are two of the most important efflux transporters that regulate the absorption, distribution, and excretion of drugs in the body. These transporters are expressed in several critical tissues, including the blood-brain barrier, liver, kidneys, and intestinal epithelium.
Since P-gp and BCRP substrates include a broad range of clinically important and structurally diverse drugs, evaluation of P-glycoprotein (P-gp) affinity is mandatory for new molecular entities and is typically conducted during preclinical development stages. One of the recommended screening methods to study tissular permeability and drug-drug interactions is through cell-based in vitro assays. Madin Darby Canine Kidney (MDCKII) cells, transfected with the MDR1 and BCRP genes, are engineered to overexpress these efflux proteins, enabling researchers to assess both MDR1 and BCRP-mediated drug transport in a single system.
Key applications of MDCK II – MDR1 and BCRP in vitro models
According to ADME specialists, MDCK II – MDR1 in vitro kits are mainly indicated for:
- Assessment of passive bidirectional transport to obtain pharmacokinetic knowledge for oral dose drug permeability in the intestinal tissue.
- Blood-brain barrier permeability diffusion to predict central nervous system metabolism.
- Evaluation of drug-efflux kinetics through the MDR1 receptor, which emulates P-gp expressing tissues’ mechanisms.
- Drug-drug interaction studies that characterize pharmacological relevant MDR1 receptor affinity.
It is crucial to include wild-type (WT) control cells in assays. WT controls act as a baseline to measure the intrinsic transport activity of the cells, providing a comparison to the overexpressed MDR1 and BCRP transporters. This enables researchers to accurately assess the specific transport mechanisms and interactions for new compounds.
Regulatory recommendations for MDR1 and BCRP evaluation
Main regulatory agencies strongly recommend to evaluate MDR1 and BCRP interactions due to their clinical importance in the absorption and disposition of drugs. Therefore, initial in vitro assessments are used to predict drug-drug interaction (DDI) potentials, aiding the development of a transporter-based clinical drug interaction strategy.
PreadyPort MDR1 and PreadyPort BCRP ready-to-use plates
ReadyCell’s product line PreadyPort-MDR1 and PreadyPort-BCRP are innovative ready-to-use cell-based in vitro systems designed for evaluating the drug-transporter interactions of new compounds. These kits optimize the drug development process, helping to prevent undesired side effects in clinical stages and streamline the transition from preclinical testing to clinical trials.
MedTech Barcelona specializes in ready-to-use in vitro plates, ideal for ADME-Tox assays, providing valuable insights into drug permeability, transporter interactions, and metabolism. For more detailed information, feel free to contact us at: reagents@medtechbcn.com.