The use of in vitro cell models, such as Caco-2 and MDCKII, is essential in biomedical research and drug development, since they provide an infinite source of reliable testing conditions before advancing to in vivo studies. However, transporting live cells has long required cryopreservation with dry ice or liquid nitrogen to preserve cell viability – solutions that are costly, logistically complex and hazardous to handle due to safety risks.
At MedTech Barcelona, we have an innovative gel-like shipping medium that enables live cell transport at ambient temperature (15-25ºC). This technology offers a cost-effective and practical alternatives to cryopreservation methods, ensuring cell viability while simplifying logistics.
Our ready-to-use cell models are shipped pre-plated, eliminating the need for cell thawing, plating, and maintaining time. This streamlined approach saves researchers time, ensures consistent experimental conditions, and reduces variability associated with traditional cell handling methods.
Globalization of scientific research through long-distance cell shipments
As scientific research expands globally, the need for safe and efficient long-distance cell transport has grown. Conventional cryogenic shipping is costly and involves safety concerns, while sending cells in liquid growth medium at room temperature is only viable for short distances, as viability significantly declines beyond 24 hours.
To address these limitations, MedTech Barcelona’s shipping medium provides a semi-solid culture system that preserves optimal moisture and protects cell integrity and functionality for up to seven days. Upon arrival, the medium can be easily liquefied at 37ºC, allowing researchers to proceed with their assays as if using freshly isolated cells.
Can the Shipping Medium preserve cell integrity during transit effectively?
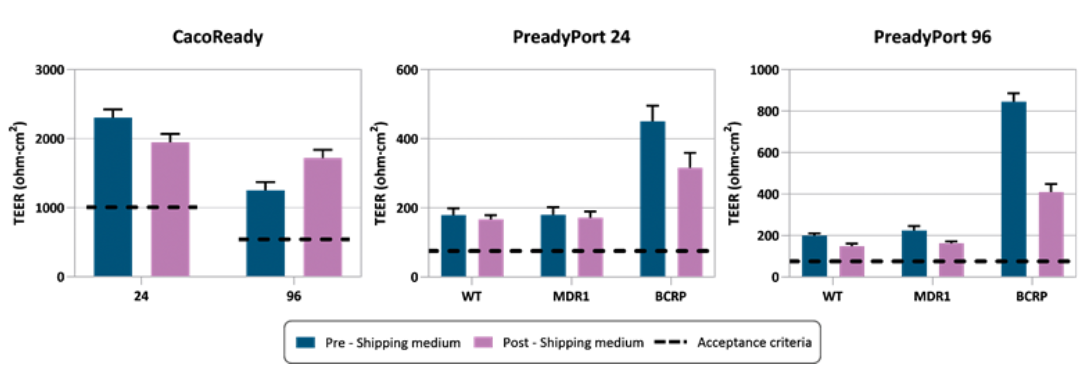
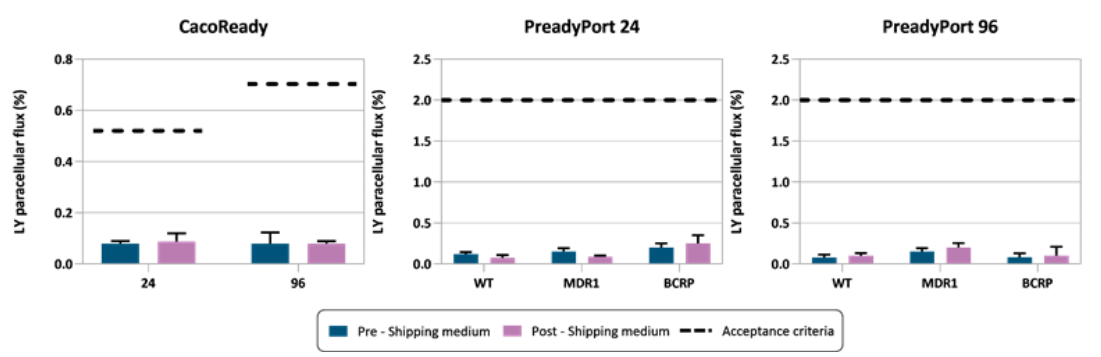
To evaluate the effectiveness of our Shipping Medium, we assess key parameters of cell barrier integrity before and after transport, such as transepithelial electrical resistance (TEER) and Lucifer Yellow (LY) permeability.
The results confirm that cells shipped in MedTech Barcelona’s shipping medium maintain TEER values comparable to pre-shipping levels confirming the preservation of their barrier function. Similarly, the results indicate that cells shipped using our medium exhibited LY permeability values similar to their pre-shipment state, demonstrating effective protection against structural damage during transit.
This patented solution ensures that epithelial cell barriers remain functionally intact throughout handling and transport, making cells immediately suitable for experimentation upon arrival. By providing a safe and efficient alternative to cryogenic shipping, our technology supports global collaboration in drug development, toxicology studies, and biomedical research.
Join the future of cell transport
At MedTech Barcelona, we are committed to advancing cell transport solutions by continually improving and optimizing our shipping medium. One of our key objectives is to enhance this technology to support the introduction of novel ready-to-use cell-based models.
If you’d like to learn more about our shipping medium and how it can support your research, please contact us through our form or at reagents@medtechbcn.com
References
- Yang, Lingzhi, et al. “An agarose-gel based method for transporting cell lines.” Current chemical genomics 3 (2009): 50.
- Wang, Junjian, et al. “Transporting cells in semi-solid gel condition and at ambient temperature.” PloS one 10.6 (2015).
- Shipping Medium® Technical File. ReadyCell. 2015
- Fabre, Myriam, et al. “Method of storing and/or transporting in vitro cell cultures.” U.S. Patent No. 8,900,842. 2 Dec. 2014.